Effective Ways to Calculate Moles in 2025: Learn More About Measurements and Units
Effective Ways to Calculate Moles in 2025: Learn More About Measurements and Units
Understanding the Mole Concept in Chemistry
The **mole concept** is fundamental in chemistry, serving as a bridge between the atomic scale and the macroscopic world. A **mole** represents a specific number of particles, typically Avogadro’s number, which is \(6.022 \times 10^{23}\) particles in one mole. This means whether you’re counting atoms, molecules, or ions, utilizing moles provides a consistent way of quantifying these entities. Understanding how to work with **mole calculations** is critical for tasks such as converting moles to grams, finding concentrations in solutions, and utilizing **molar mass** during experiments. Chemists frequently use the **mole formula** to derive relationships between numbers of particles and the mass of substances. Grasping the fundamental principles of the mole concept is crucial for any aspiring chemist.
Significance of Moles in Chemical Reactions
Moles play a significant role in chemical reactions, allowing scientists to predict how substances will interact. Understanding **mole relationships** helps in determining reactant and product quantities using stoichiometry. For example, when balancing a chemical equation, knowing the **mole ratios** between reactants and products allows chemists to calculate the number of moles needed for a reaction. When conducting these calculations, it’s essential to remember the significance of the **mole in stoichiometry**, which ties directly to how **moles in chemical equations** can dictate yield and efficiency. Accurate mole calculations not only streamline reactions but also enhance safety standards in laboratory practices.
Calculating Moles from Mass and Molar Mass
To convert mass to moles, you need to know the **molar mass** of the substance. The formula for calculating moles from mass is given as:
\[ \text{Number of Moles} = \frac{\text{Mass of Substance (g)}}{\text{Molar Mass (g/mol)}} \]
For example, if you have 18 grams of water (with a molar mass of approximately 18 g/mol), you can calculate the number of moles by dividing the mass of water by its molar mass. Thus, you have 1 mole of water. Understanding this concept can aid in various aspects of **calculating moles from mass**, enabling efficient measurement practices in both academic and professional settings.
Mole Conversion Techniques
Mastering **mole conversion** is essential for facilitating various chemical calculations. Part of this mastery includes knowing how to switch between moles, grams, and liters. For example, a chemist often needs to convert **grains to moles** and vice versa. This can be achieved through the utilization of molar mass and Avogadro’s number, even in diverse applications involving **moles in solutions** and their corresponding concentrations. By implementing dimensional analysis, you can simplify complex conversion errors and streamline the learning process when working with moles. Whether in a laboratory or a theoretical setting, effective conversion techniques remain vital.
Conversions with Moles to Grams
Conversions from moles to grams follow a straightforward process. The **molar mass** serves as the conversion factor between mass and the number of moles. Let’s consider a practical example: if you need to find the mass of 2 moles of carbon dioxide (CO₂), knowing the molar mass of CO₂ (approximately 44 g/mol) allows you to perform the conversion as below:
\[ \text{Mass of CO}_2 = 2 \, \text{moles} \times 44 \, \text{g/gmol} = 88 \, g \]
Such conversions are vital in many aspects of chemistry, including precise formulations in laboratory settings and during chemical production processes.
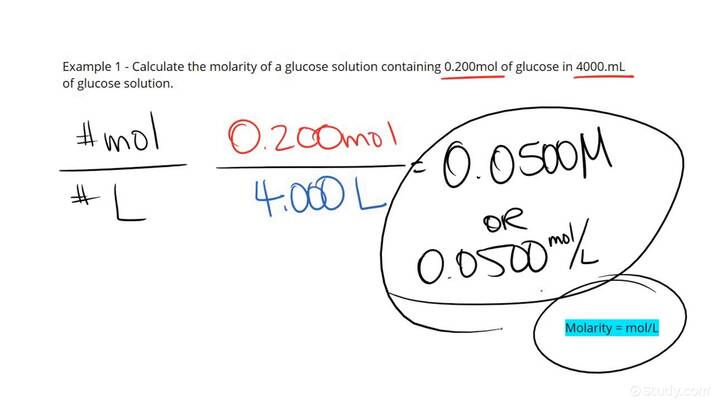
Molarity and Moles in Solutions
Molarity, defined as the moles of solute per liter of solution, enables chemists to accurately prepare and analyze solutions. Understanding the relationship between **moles and solutions** is crucial for various applications such as dilution and titration. The formula for calculating molarity is expressed as:
\[ \text{Molarity (M)} = \frac{\text{Moles of Solute}}{\text{Liters of Solution}} \]
For instance, to make a 1 M solution of sodium chloride (NaCl), one would need to dissolve 1 mole of NaCl in 1 liter of water. Realizing the concept of **molarity and moles** streamlines the process of preparing chemical solutions tailored to particular uses.
Calculating Moles from Density
Calculating moles from density involves understanding the relationship between mass, volume, and density. The formula connects these three essential factors:
\[ \text{Density} = \frac{\text{Mass}}{\text{Volume}} \]
Using this formula, you can rearrange it to determine the mass of a substance using its density and volume. Following a similar path, once you find mass, converting to moles becomes a simple application of the mole formula. This technique is particularly useful for dealing with substances in their gaseous states, where ideal gas law can also come into play. In addition, it allows the conversion of different states of matter to determine how many **moles from density** are present.
Finding Moles of Gas Using the Ideal Gas Law
The ideal gas law, \( PV = nRT \), is pivotal in calculating the number of moles of a gas under specific conditions of pressure and temperature. Here, \( P \) stands for pressure, \( V \) for volume, \( n \) for the number of moles, \( R \) for the ideal gas constant, and \( T \) for temperature. This equation allows chemists to determine how many moles of gas exist within a particular volume at given conditions. For instance, if you know the pressure is 1 atmosphere, the volume is 22.4 liters, and the temperature is standard at 273.15 K, you can calculate the moles present effectively through this relationship. This aspect of **calculating moles of gas** significantly enhances research and application in physical chemistry and related fields.
Moles and Chemical Reactions
Moles are crucial in stoichiometry for predicting outcomes in chemical reactions. Using stoichiometric coefficients in balanced chemical equations assists chemists in determining the number of moles of reactants and products. For example, if a reaction states that 2 moles of hydrogen gas react with 1 mole of oxygen gas to produce 2 moles of water, understanding the **mole ratios** makes predicting the yield straightforward. This knowledge not only helps in grasping the fundamental **mole calculations** adjust to various situations in chemical reactions but also supports efficient resource allocation and safety protocols within laboratory environments.
Key Takeaways
- Understanding the mole concept is foundational in chemistry for quantifying particles.
- Effective conversion techniques between moles, mass, and volume enhance precision in measurements.
- Calculating moles from various references such as density adds value to analysis in chemistry.
- Moles are indispensable in chemical reactions, dictating outcomes through stoichiometric behaviors.
- Practicing mole calculations aids in mastering essential chemistry concepts for practical applications.
FAQ
1. What is the importance of Avogadro’s number in mole calculations?
Avogadro’s number, \(6.022 \times 10^{23}\), is central to understanding **how to find the number of moles** as it represents the number of particles in one mole of a substance. This concept is essential for converting between moles, atoms, and molecules, allowing for accurate calculations in stoichiometry and chemical reactions.
2. How can moles be converted into liters for solutions?
To convert moles into liters, you need to understand molarity. The formula is:
\[ \text{Liters} = \frac{\text{Moles}}{\text{Molarity}} \]
By knowing the number of moles and the desired molarity, you can determine how many liters of solution can be made, aiding in practical applications in chemistry lab settings.
3. How do you determine moles from density calculations?
To determine moles from a density calculation, use the relationship:
1. Calculate mass from density:
\[ \text{Mass} = \text{Density} \times \text{Volume} \]
2. Then convert mass to moles:
\[ \text{Moles} = \frac{\text{Mass}}{\text{Molar Mass}} \]
This approach enables a comprehensive understanding of calculating moles in various states of matter.
4. What are the practical applications of moles in everyday life?
Moles have numerous applications in everyday life, such as determining the amount of ingredients in cooking, analyzing concentration in water quality assessments, and calculating dosages in medicine and pharmacology. They hold relevance in various fields such as environmental science, biology, and agriculture.
5. How are moles linked to the ideal gas law?
Moles are integrally linked to the ideal gas law \( PV = nRT \), where \( n \) represents the number of moles. This relationship is crucial in chemistry for calculations involving gases, as it provides a means to predict behavior under various conditions of pressure and temperature.
6. What common challenges do students face when learning about moles?
Students often face challenges such as grasping the abstract concept of a mole, performing conversions correctly, and applying stoichiometry effectively in chemical equations. Overcoming these challenges requires practice, guided examples, and ideally, hands-on laboratory experiences.
7. What role do moles play in calorimetry?
Moles are essential in calorimetry as they provide a measurement to calculate energy changes in chemical reactions involving heat transfer. By quantifying reactants in moles, one can accurately analyze reaction enthalpy and thermodynamic properties vital in physical chemistry.