Smart Ways to Find the Limiting Reactant in Your 2025 Chemistry Lab!
Smart Ways to Find the Limiting Reactant in Your 2025 Chemistry Lab!
Finding the limiting reactant is a fundamental concept in chemistry that is crucial for understanding how chemical reactions progress. In any chemical reaction, one reactant is often consumed before the others, limiting the amount of product formation that can occur. This article explores effective methods to determine the limiting reactant and offers practical insights you can utilize in your 2025 chemistry lab experiments.
Understanding Limiting Reactants
The concept of the limiting reactant is vital in stoichiometry. It refers to the reactant that will be completely consumed first, thereby stopping the entire reaction. This understanding is essential for optimizing reaction yields and minimizing waste. Whenever you conduct a reaction, knowing which reactant limits the reaction enables you to predict how much product will be formed based on the initial quantities of your reactants.
The Role of Balanced Equations
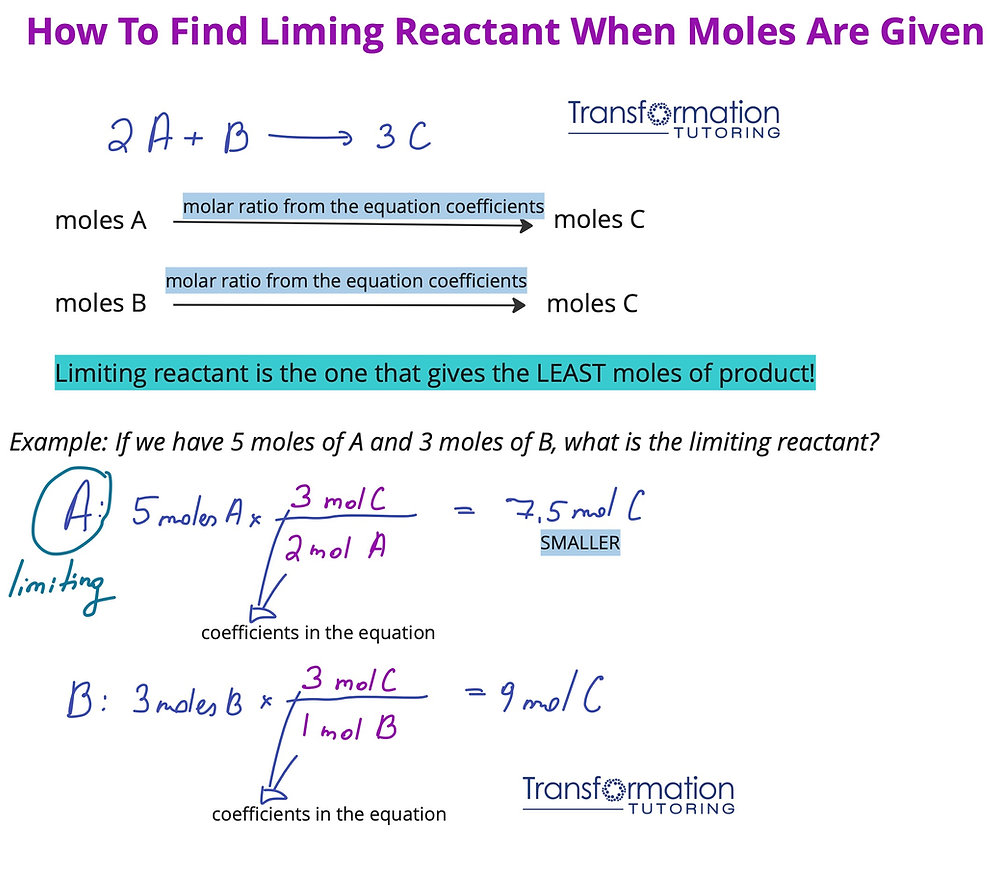
To determine the limiting reactant, you must start with a balanced chemical equation. Each reactant’s coefficients represent the mole ratio needed for the reaction. For example, in the equation for the combustion of propane, C₃H₈ + 5O₂ → 3CO₂ + 4H₂O, the coefficients indicate that one mole of propane reacts with five moles of oxygen. Analyzing these coefficients allows you to calculate how much of each reactant is needed for complete reaction, leading to accurate reactant calculations.
Steps to Identify the Limiting Reactant
Identifying the limiting reactant requires systematic steps:
- Balance the chemical equation.
- Convert the amounts of all reactants to moles, using their respective molar mass.
- Use the mole ratio from the balanced equation to compare the amounts of the reactants.
- The reactant that produces the least amount of product is the limiting reactant.
Following these steps effectively enables you to conduct quantitative analysis of your chemical reactions, ensuring you understand both reactant proportions and expected yields.
Example Calculation
For instance, consider a reaction where 2 moles of hydrogen react with 1 mole of oxygen: 2H₂ + O₂ → 2H₂O. If you start with 3 moles of hydrogen and only 1 mole of oxygen, you can convert the amounts to determine which reactant is limiting. Based on the mole ratios from the balanced equation, you would need 1.5 moles of oxygen to react completely with the available hydrogen. Since you only have 1 mole of oxygen, it is the limiting reactant.
Measuring Excess Reactants
After identifying the limiting reactant, you can also analyze the excess reactant. This information helps in refining your reaction process for future experiments, allowing you to use chemicals more efficiently and reduce waste. Knowledge of the excess reactants also leads to an enhanced understanding of the completeness of the reaction, vital for calculating theoretical vs practical yields.
Quantifying Excess Reactants
To quantify the excess reactant, first, determine how much of it will be used based on the limiting reactant’s amount. Taking the previous example, if you know oxygen is limiting and 1 mole is consumed to make water, you can calculate how much hydrogen remains. If you started with 3 moles of hydrogen, you would have 2 moles left after the reaction, showcasing efficient reactant efficiency and management through proper chemical calculations.
Safety and Best Practices in Chemical Reactions
When working in a lab, it’s essential to adhere to safety protocols. This includes proper handling of chemicals and measuring reactants accurately to prevent dangerous reactions. Ensure all appropriate laboratory safety equipment is worn and that you follow best practices, from starting with small quantities to properly labeling all containers to minimize the risks associated with chemical reactions.
Applications of Limiting Reactant Concepts
Understanding limiting reactants serves various applications beyond just the laboratory. It is crucial in real-world processes such as industrial chemical production, where maximizing efficiency translates directly to cost savings. Additionally, this fundamental principle is applied in educational settings to deepen the understanding of chemistry principles.
Applications in Industry
In industrial applications, producers depend on accurate limiting reactant determinations for large-scale synthesis. For instance, in the manufacture of ammonia through the Haber process, understanding the stoichiometry ensures optimal input of nitrogen and hydrogen, ultimately affecting profitability and sustainability.
Implications in Research and Development
In research and development, identifying limiting reactants fosters the innovation of new chemical processes and materials. Scientists rely on this knowledge when developing pharmaceuticals, materials science, and environmental technology, allowing for more efficient reaction mechanisms and exploration of chemical pathways.
Teaching and Learning Chemistry
In educational contexts, teachers effectively use the concept of limiting reactants to engage students with practical applications. Introducing limiting reactant examples and supporting students in hands-on experiments enhances their grasp of important chemistry concepts.
This practice empowers future scientists to innovate chemical practices meaningful to society.
Key Takeaways
- Understanding limiting reactants is crucial for optimizing chemical reactions.
- Careful measurements and stoichiometric calculations lead to accurate outcomes.
- Real-world applications can benefit from efficient management of reactants.
- Safety practices in the lab ensure reactants are handled properly.
FAQ
1. What is a limiting reactant in a chemical reaction?
A limiting reactant is the substance in a chemical reaction that is used up first, preventing the reaction from continuing and determining the total amount of product that can be formed.
2. How do balanced equations help identify a limiting reactant?
Balanced equations provide the necessary mole ratios that allow you to compare the amounts of reactants you have. By knowing how each reactant relates to the others in terms of quantity needed, you can identify the limiting reactant through stoichiometric calculations.
3. Can you give an example of how to calculate a limiting reactant?
Sure! For example, in the reaction of 4H₂ + O₂ → 2H₂O, if you start with 6 moles of hydrogen and 1 mole of oxygen, you would convert given amounts into ratios using the balanced equation. In this case, oxygen would be the limiting reactant since only 2 moles of hydrogen would be required for complete reaction with the 1 mole of oxygen available.
4. Why is identifying excess reactants important?
Identifying excess reactants is essential for resource management. Knowing how much of a reactant is unreacted allows for better control in chemical calculations, thus reducing waste, improving sustainability, and potentially lowering costs in industrial applications.
5. What safety measures should I consider in the laboratory while working with limiting reactants?
Safety always comes first! Always wear personal protective equipment (PPE), such as gloves and goggles. Properly label all chemicals, follow handling procedures, and be precise in your measurements to ensure minimal reactions pose risks of spills or accidents. Additionally, having appropriate safety data sheets (SDS) readily available in the lab can help in handling emergencies related to chemical contact.
By understanding limiting reactants, you can take your chemistry lab skills to the next level. Implement these strategies and see how they apply in your experimental processes!